Mastering Study Import & Deduplication
Learn how to efficiently import thousands of studies from various sources like PubMed and Scopus, and use RevPro’s powerful tools to deduplicate them.
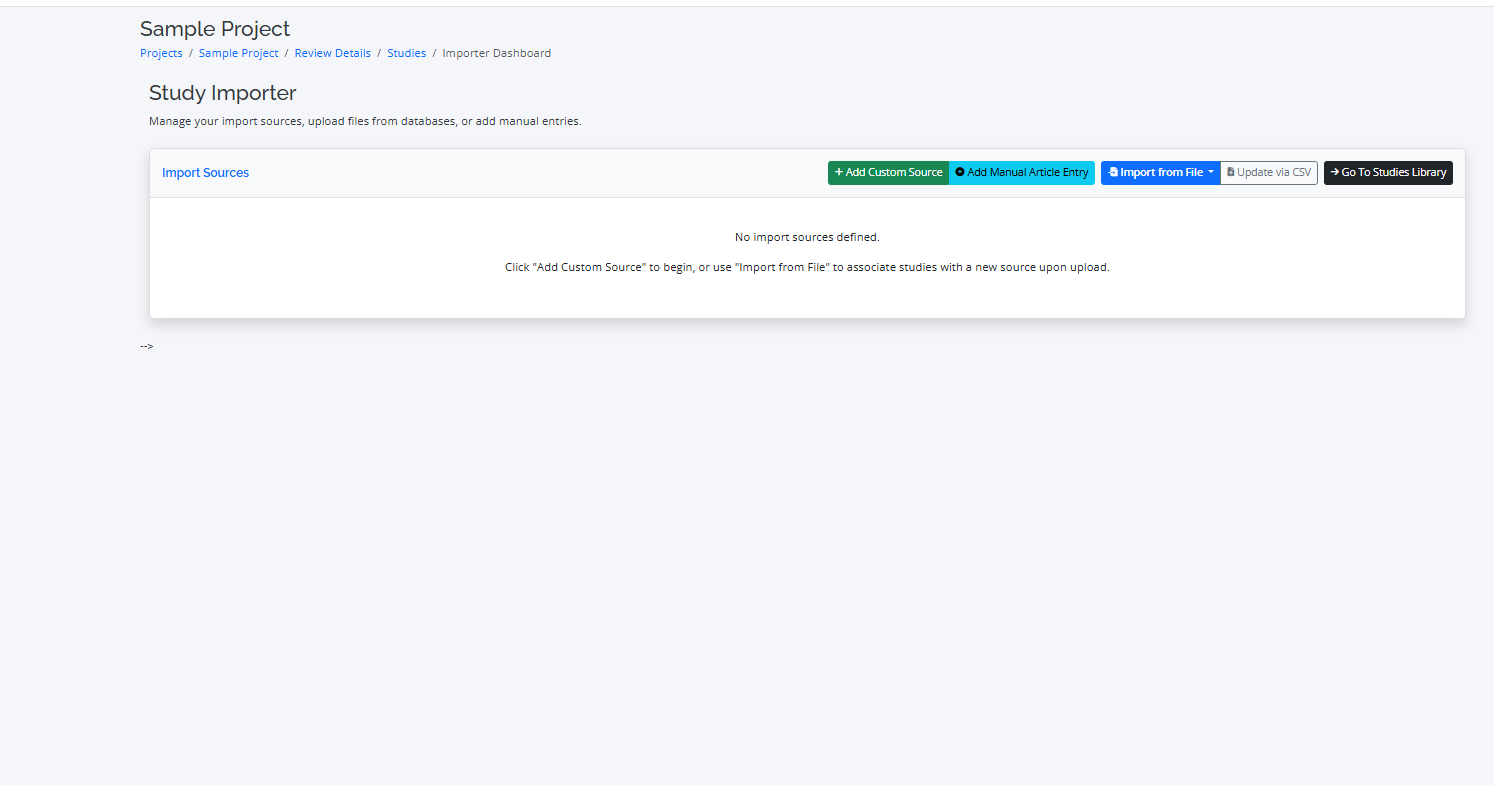
Populating Your Project with Studies
The first step in conducting your review is to import the studies identified from your database searches. RevPro makes this process simple and efficient.
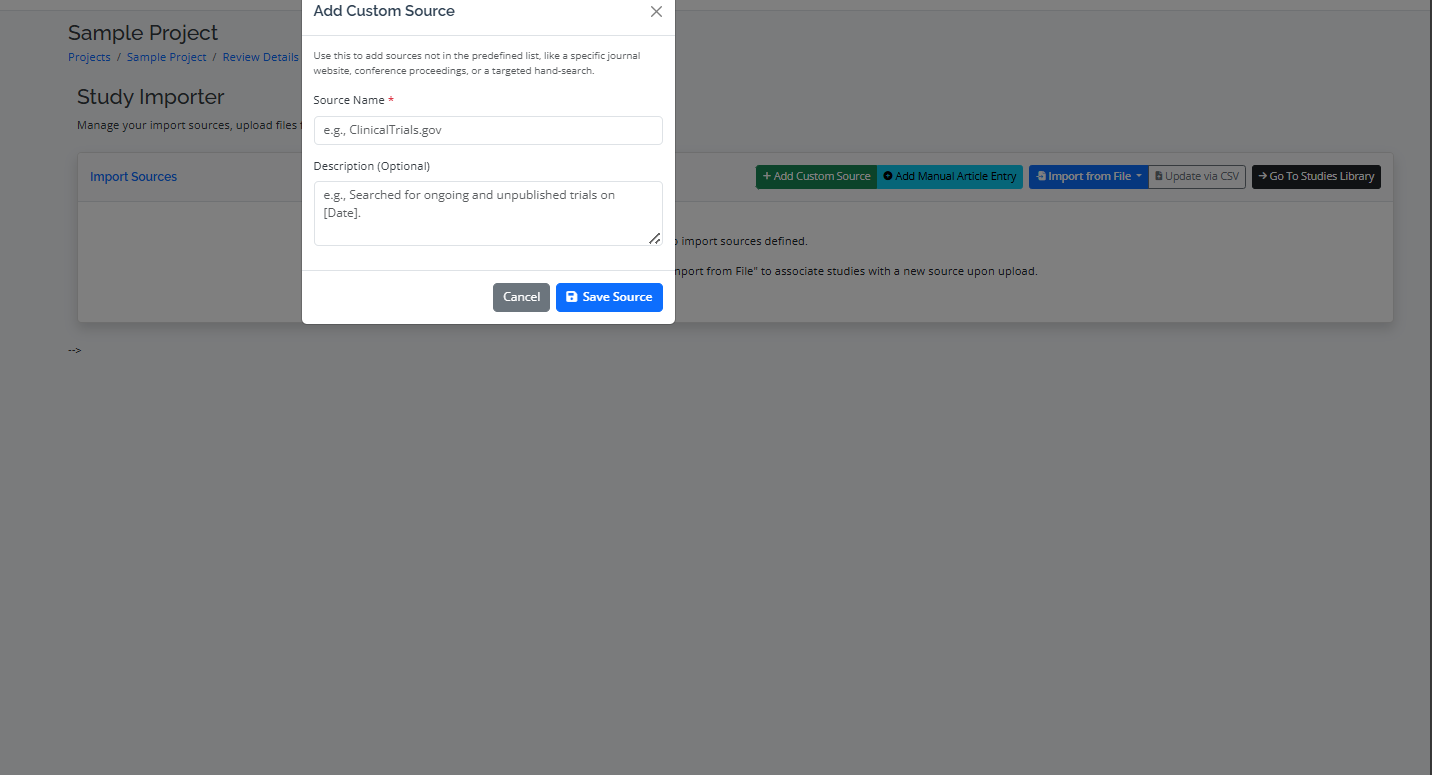
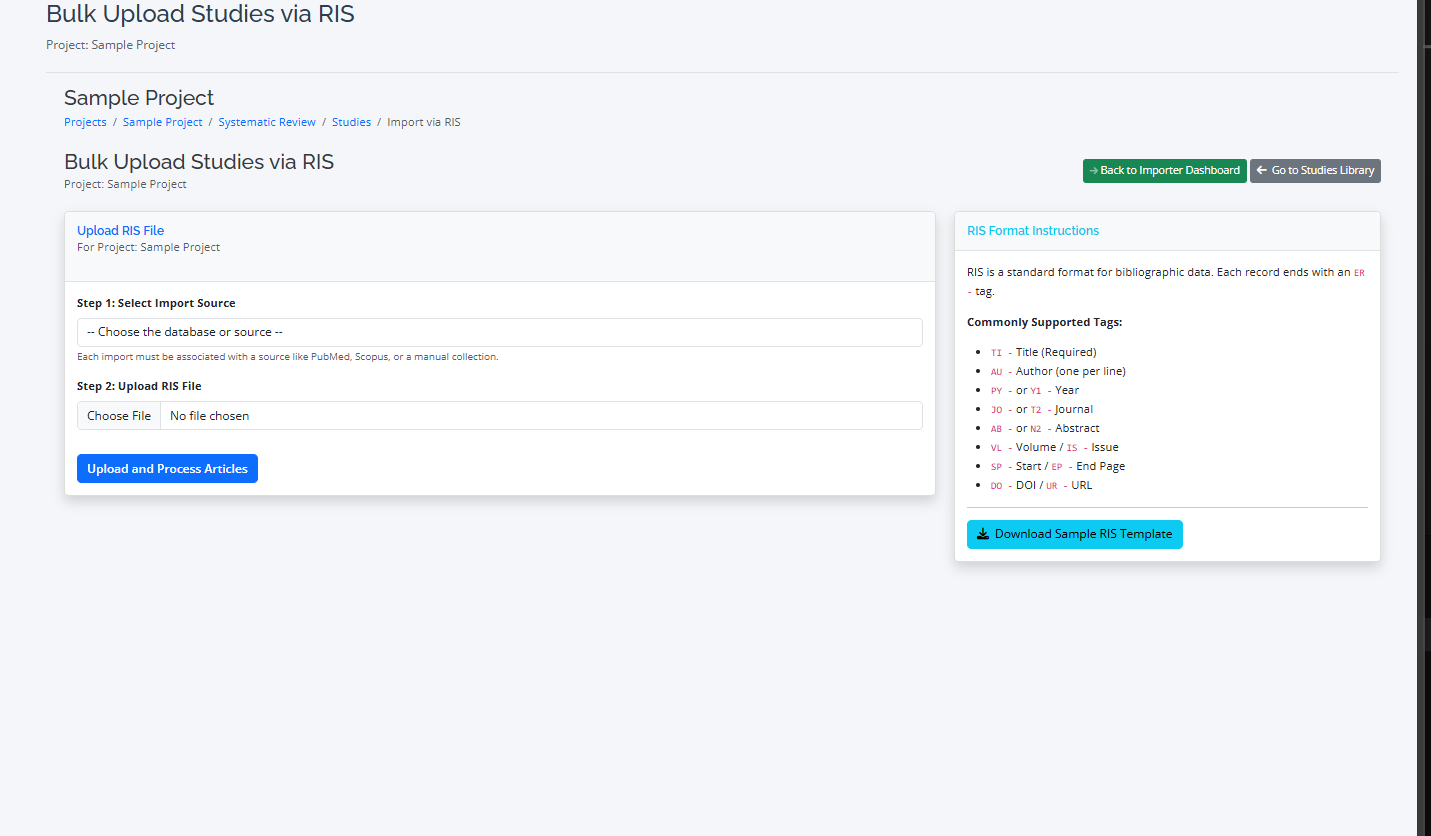
Step 1: Importing from Different Sources
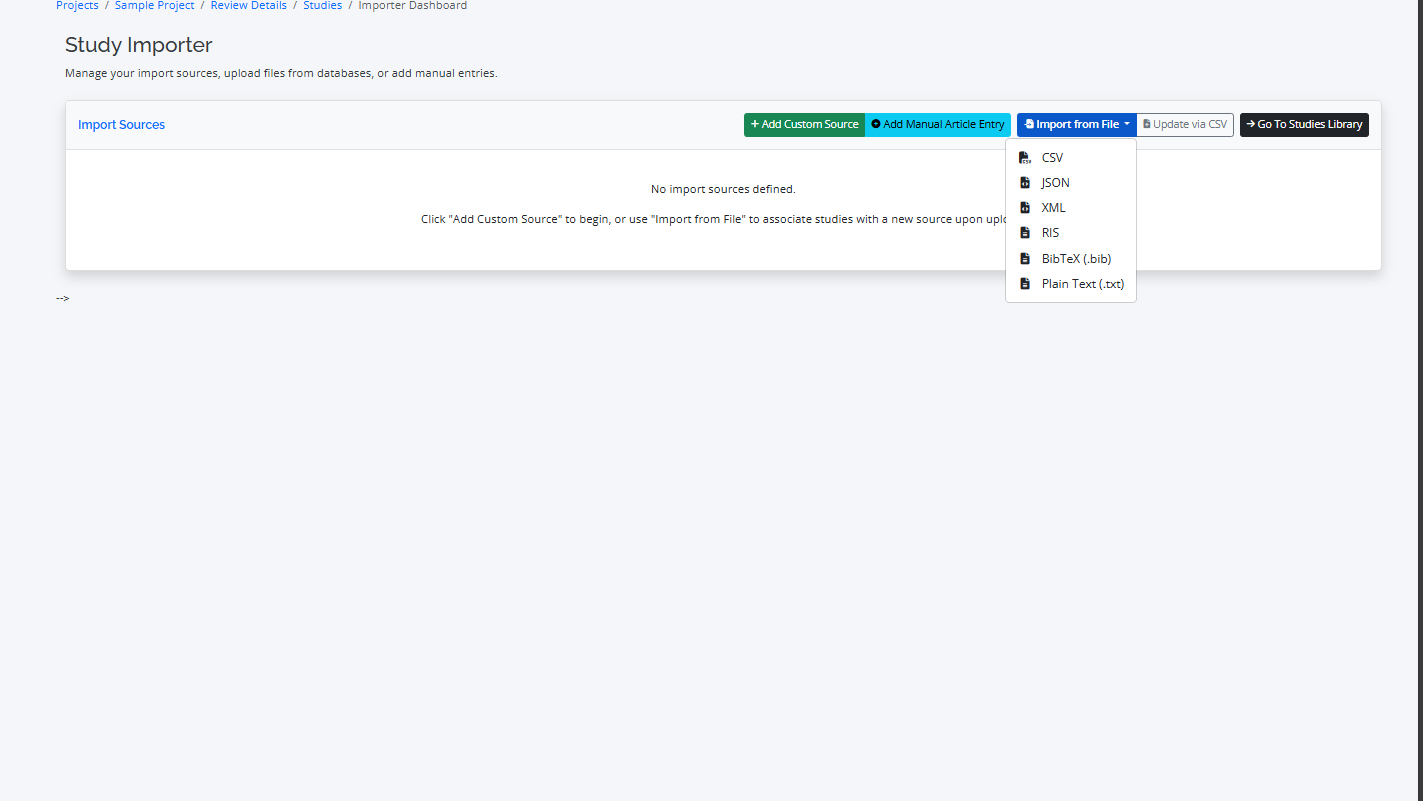
RevPro supports common file formats from major academic databases.
- Supported Formats: CSV, RIS, BibTeX, XML, JSON, TEXT
- Import Process: Go to the "Import" tab, select your source database (e.g., PubMed, Scopus), and upload the file. RevPro will automatically parse the file and import the studies.

Step 2: The Deduplication Process
Searching multiple databases inevitably leads to duplicate entries. RevPro's deduplication tool helps you clean up your study list with ease.
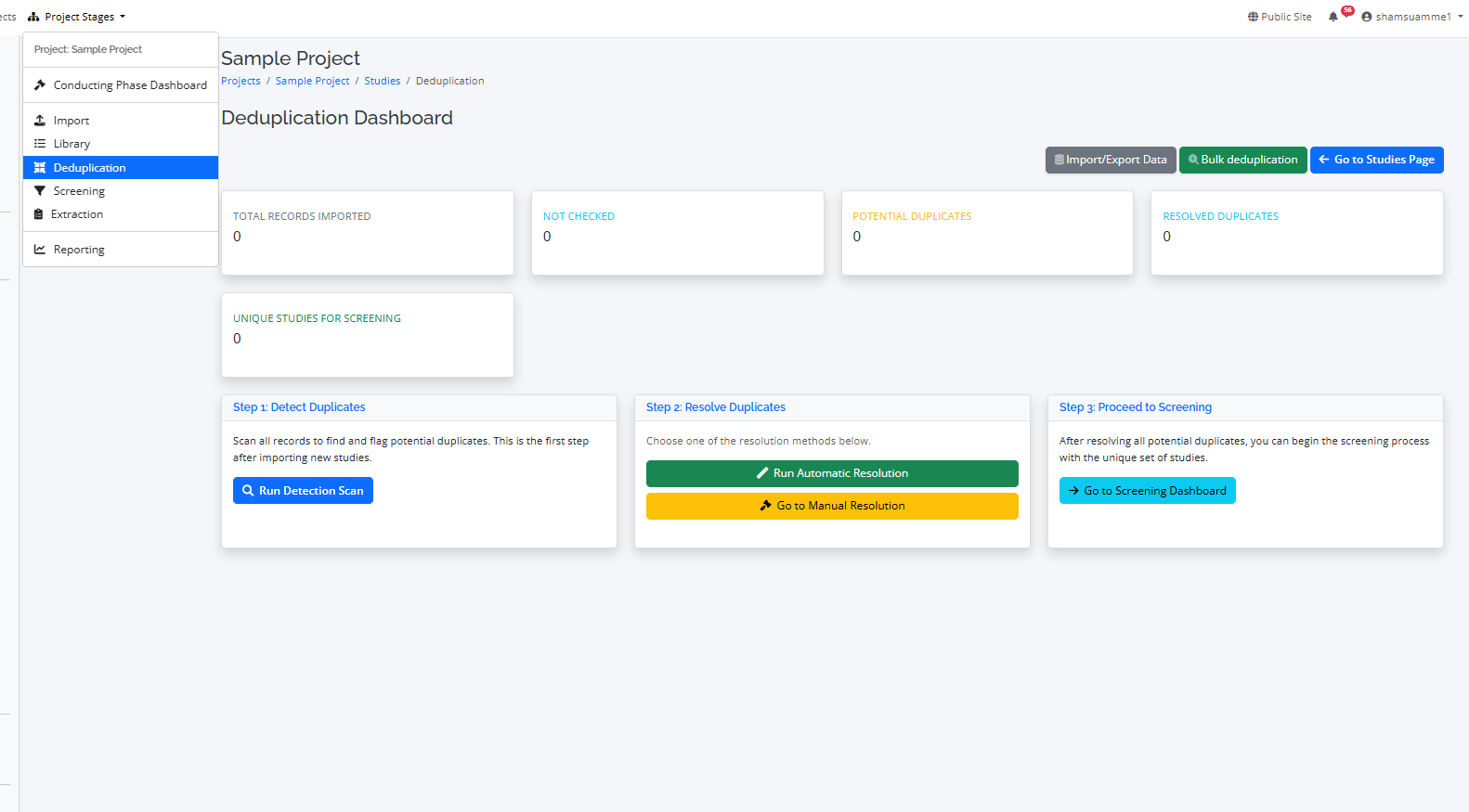
- Automatic Detection: RevPro uses an advanced algorithm to automatically identify potential duplicates based on titles, authors, and other metadata.
- Automatic and manual deduplication: Choose one of the resolution methods below.
- Manual Review: A dedicated interface allows you to review potential duplicates side-by-side and make the final decision to merge or keep them separate.
- Automatic resolution: A dedicated function to process groups, resulting in unique studies and resolved duplicates.
- Bulk deduplication: A dedicated interface allows you to remove potential bulk duplicates, make or update the final decision.
- Audit Trail: All deduplication decisions are logged, maintaining the integrity of your review process.
Bulk deduplication
A dedicated interface allows you to remove potential bulk duplicates, make or update the final decision.

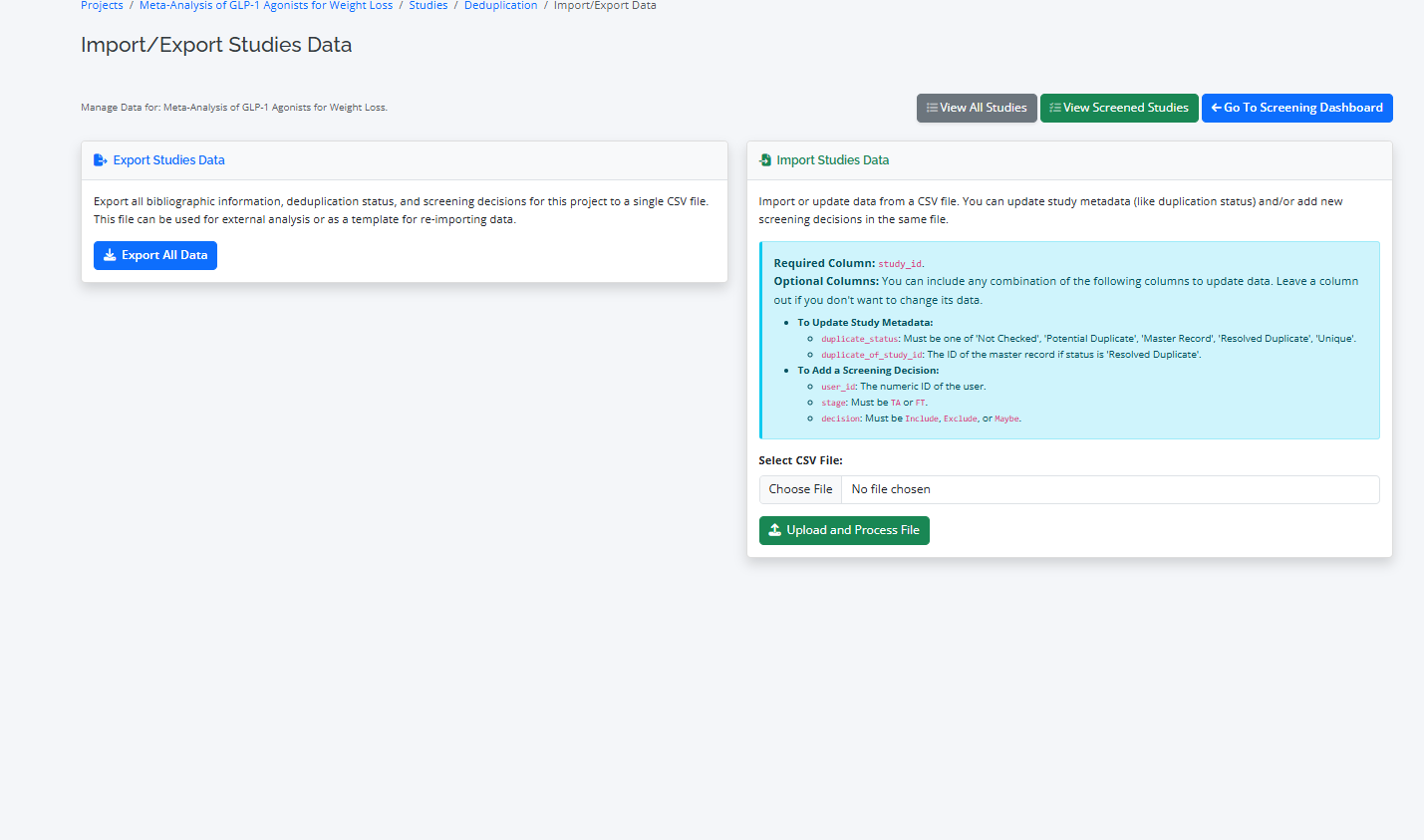
Export and Import Studies Data
Export all bibliographic information, deduplication status, and screening decisions for this project to a single CSV file. This file can be used for external analysis or as a template for re-importing data.
You can import updates to the studies using csv format.